QUA’s FEDI®-Rx proved a perfect retrofit for other manufacturer’s EDI stacks and has been delivering high purity, pharmaceutical grade water consistently for the last 4 years, irrespective of variation in feed water conditions.
Background
The clients laboratory has been providing quality back-end support to the pharmaceutical industry at large since its inception in 1988. Located at Pithampur, India, the laboratory has been committed to strategic research and delivering a complex range of chemistries that are globally benchmarked.
The client had an existing purified water system to produce USP grade water with conductivity less than 1 μS/cm. The system consisted of a softener, reverse osmosis system and electrodeionization (EDI) as the final polisher to achieve water conductivity as per USP requirement.
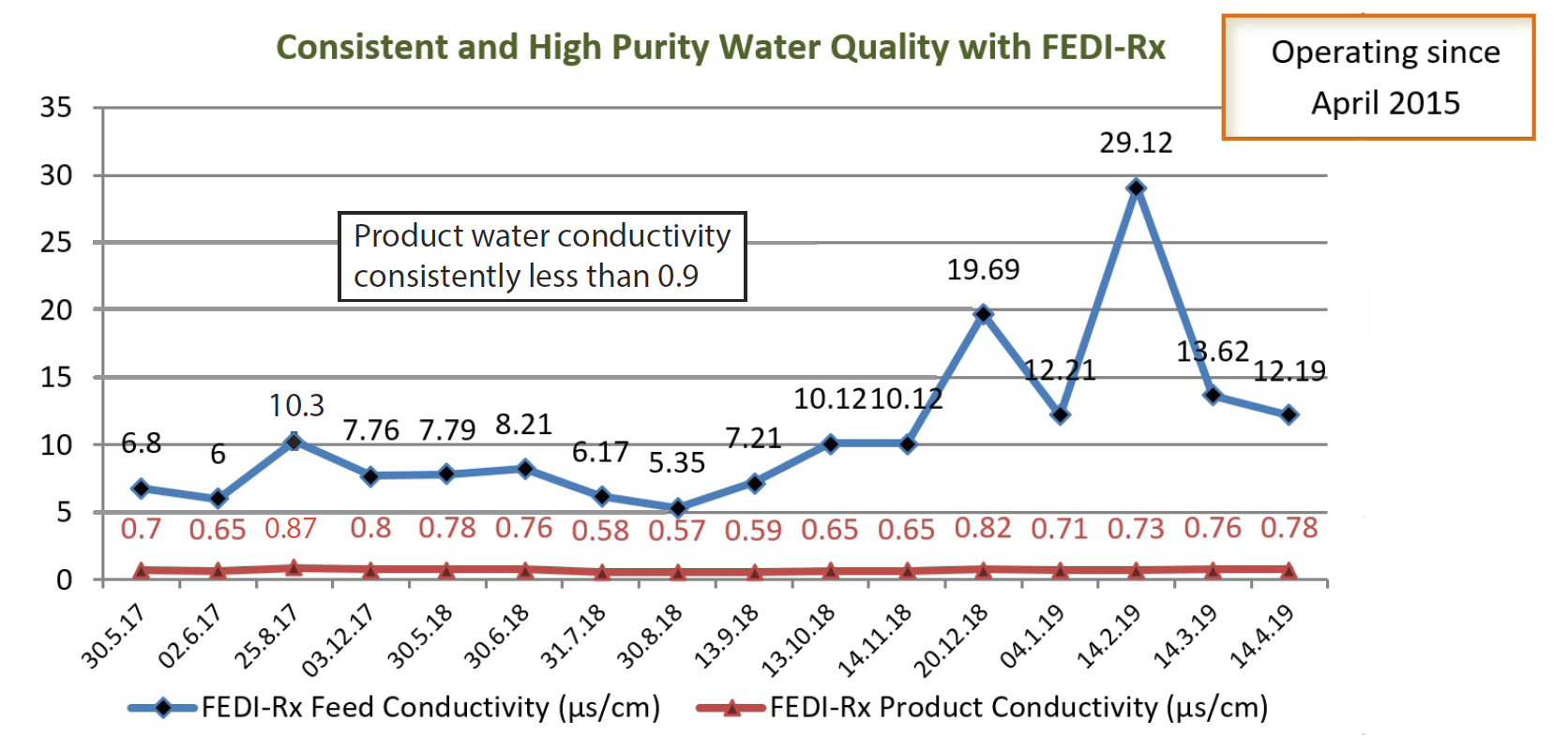
The client’s existing electrodeionization system, with another manufacturer’s EDI, was not able to deliver consistent product water quality due to variable feed water conditions. The feed water quality was fluctuating, with conductivity at times close to 30 μS/cm. As a result their production was adversely affected and they started exploring other EDI options. The client required
- An EDI which could deliver consistent product water quality on a continuous basis irrespective of variation in feed water conditions.
- An EDI that would be an easy retrofit to replace the existing unit.
- They wanted the EDI with least lead time for delivery.
Additionally, service was a key issue as the customer was not satisfied with the existing EDI manufacturer’s support, and expected their new EDI supplier to understand the problem and work closely to resolve quality concerns as soon as possible.
FEDI Model: FEDI-2-10Rx
FEDI Stack: 1
Flow: 0.85 m3/hr
Application: USP grade water for pharmaceutical application
QUA Solution
After a detailed technical evaluation, the OEM and the client selected QUA’s FEDI®-2-10Rx as an easy retrofit solution. FEDI-Rx series stacks are typically used in USP grade purified water/WFI systems. These stacks can be hot water sanitized at 85°C. The wetted components of FEDI-Rx stacks conform to US FDA requirements. The stacks are CE certified and come with a triclover end connections. FEDI-Rx stacks can produce water quality upto 18 megaOhms.
Since delivery schedule was critical to ensure uninterrupted pharma production, QUA delivered FEDI-Rx within one weeks’ time. All inputs related to retrofit were provided to the OEM which enabled them to complete the retrofit activity within 2 days.
QUA’s dedicated technical support team provided outstanding pre and post-sales support to the client and was present at site throughout commissioning. The client’s operation team was given comprehensive training on the operation of FEDI-Rx, and troubleshooting. QUA has closely been monitoring the site along with the client’s operations team, to ensure trouble free operation of FEDI.
The FEDI-Rx module was commissioned in April 2015, and has been delivering consistently high purity water of conductivity less than 1 microS/cm for the last 4 years.
About FEDI
The Fractional Electrodeionization (FEDI) process is an advancement of EDI, and was developed taken into account the limitations of conventional EDI. FEDI’spatented Dual Voltage process allows for higher flexibility and tolerance to inlet water conditions, thus lowering the risk of scaling, and improving the plant’s design economics and reliability.
FEDI’s patented dual voltage technology ensures:
• A two-stage separation process that achieves a higher hardness tolerance
• Better Silica rejection
• Optimized power consumption
• The best water quality, continuously and consistently
Read more.





