
FEDI High Flow stack for High Purity water for Pharmaceutical application
QUA’s FEDI HF stack delivers high purity water with a lower footprint for the pharmaceutical manufacturer.
Project Background
A pharmaceutical manufacturer’s plant required high purity water for manufacturing of their range of various pharmaceutical, cosmetic, and nutraceutical products. The manufacturer selected a process scheme for a high purity water system, comprising of pretreatment followed by two pass reverse osmosis (RO) followed by electrodeionization (EDI). The required high purity water system capacity was 5m3/hr. At this flow rate, the requirement called for two EDI stacks configuration in parallel operation.
FEDI Model: FEDI-2HF-30X
Flow: 5 m3/hr (22 gpm)
No. of Stacks: 1
Conductivity: <0.5 µS/cm
Application: High purity water for pharmaceutical application
QUA Solution
Due to a higher capital cost required for using a two EDI stack configuration, the client was looking for the optimal solution that would accommodate the use of a single EDI stack to deliver a high flow rate. After detailed technical and commercial evaluation, QUA’s FEDI-2-HF-30X stack was selected for the application due to its ability to deliver high flow rate in a single stack configuration, resulting in capex savings for the client.
QUA’s Fractional Electrodeionization (FEDI) high flow (HF) stacks are designed for operation after double pass reverse osmosis. The stack has the ability to produce high purity water at a high flow rate using a patented “Split Flow EDI” technology. The salient features of the FEDI HF stacks are:
- High stack flow up to 8.4 m3/hr (37 gpm)
- Lower capex costs due to reduction in footprint
- Low operating costs
- High recoveries
- Superior product quality up to 16 MOhms.cm
- Meet water specifications for various high purity water applications
QUA supplied a single FEDI-2HF-30X stack for this application requiring 5 m3/hr of product flow.
Results
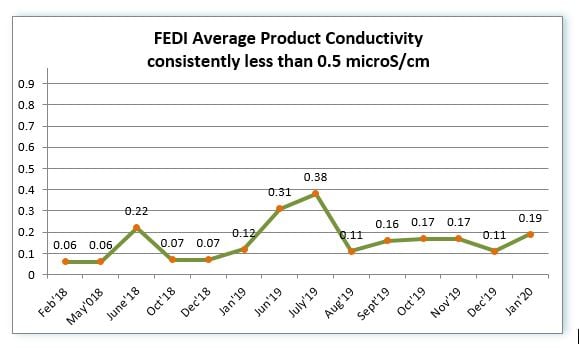
The FEDI high flow stack has been in operation since December 2016 on a continuous basis. The product water quality has been consistently less than 0.5 microS/cm, which meets the customer’s requirement. The following graph shows the product water conductivity trend from 2018 to 2020. QUA’s FEDI successfully provides a consistent high purity water solution for the pharmaceutical manufacturer’s process requirements.
About QUA
QUA is an innovator of advanced membrane technologies that manufactures and
provides filtration products to address the most demanding water challenges.
FEDI® Electrodeionization
QUA’s Fractional Electrodeionization (FEDI) is an advancement of the EDI technology, that was developed to address the limitations of conventional EDI. FEDI is a patented two stage process that operates in a dual voltage configuration that reduces hardness scaling that may occur in Product Conductivity Consistently Less than 0.2 microS/cm conventional EDI. FEDI’s unique design maintains an acidic condition in the first stage and basic condition in the second stage of the EDI concentrate chamber. This patented design reduces mineral scaling in the first stage and enhances silica removal in the second stage.
Click here to download :https://quagroup.com/wp-content/uploads/FEDI-N-Pharma.pdf

